CELLnTEC’s Higher Certified (HC) products
Higher Certified (HC) culture media by CELLnTEC are used in translational bench-to-bedside research projects. They enable a seamless transition from the development of cell-based therapies into clinical trials.
HC culture media are manufactured using upgraded ingredients and processes and are successfully being used in a range of Phase I and II clinical trials. These media are ideally suited for use during early stages like product development, protocol validation, and characterization of the cell-based product before entering clinical trials. Utilization of HC media for the early phases ensures:
-
- All processes and products are finalized using the identical medium formulation that will be used during the clinical trials
- Sources of variability are eliminated
- Re-validation of protocols following a change in culture medium ingredients is not required
- All data submitted for regulatory approval are obtained using the identical products and processes that will be used clinically
Highlights of HC media
Higher Certified (HC) culture media differ from CELLnTEC’s standard research-grade media in the following ways, to meet progressing user needs with respect to the following criteria:
| COMPONENTS | Clinically compatible, well documented, tight tolerances. |
| MANUFACTURING | Tight documentation, dedicated equipment, additional process certification. |
| QC PROCESS | Additional Quality Control testing steps for all HC media. |
| DOCUMENTATION | Extended information regarding component origin, manufacturing processes, chemical composition. |
| CONSISTENCY | Product formulation will remain consistent. No changes will be made without prior notification to the end user. |
| SUPPORT | Additional range of services, including custom product documentation, advanced technical & scientific support, support with regulatory processes, lot reservation. |
| SERUM FREE | HC media are chemically defined and serum-free *. |
* The Higher Certified (HC) versions are supplied without serum supplementation for those products that do require serum in their standard research versions.
This enables the end-user to supplement with serum validated to deliver the desired cell behaviour and known to meet the necessary regulatory requirements.
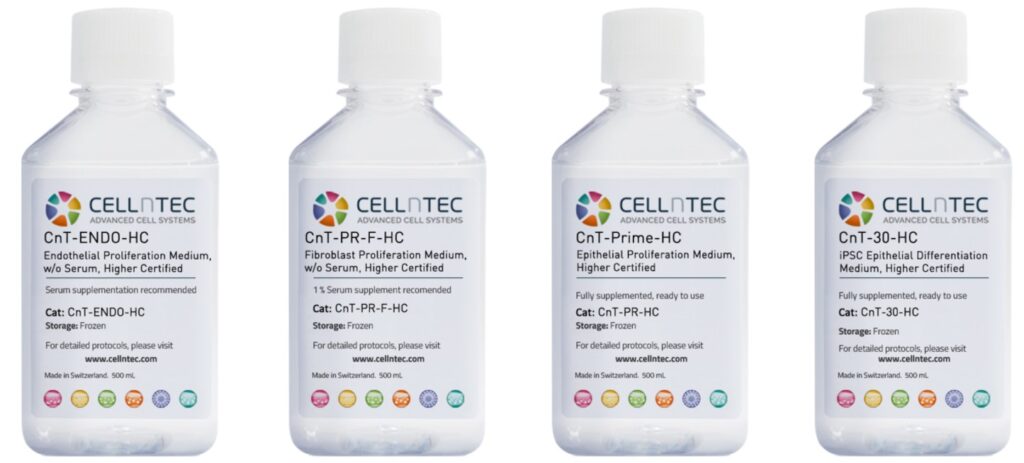
CELLnTEC’s HC media portfolio
For more information and quotes contact techsupport@caltagmedsystems.co.uk