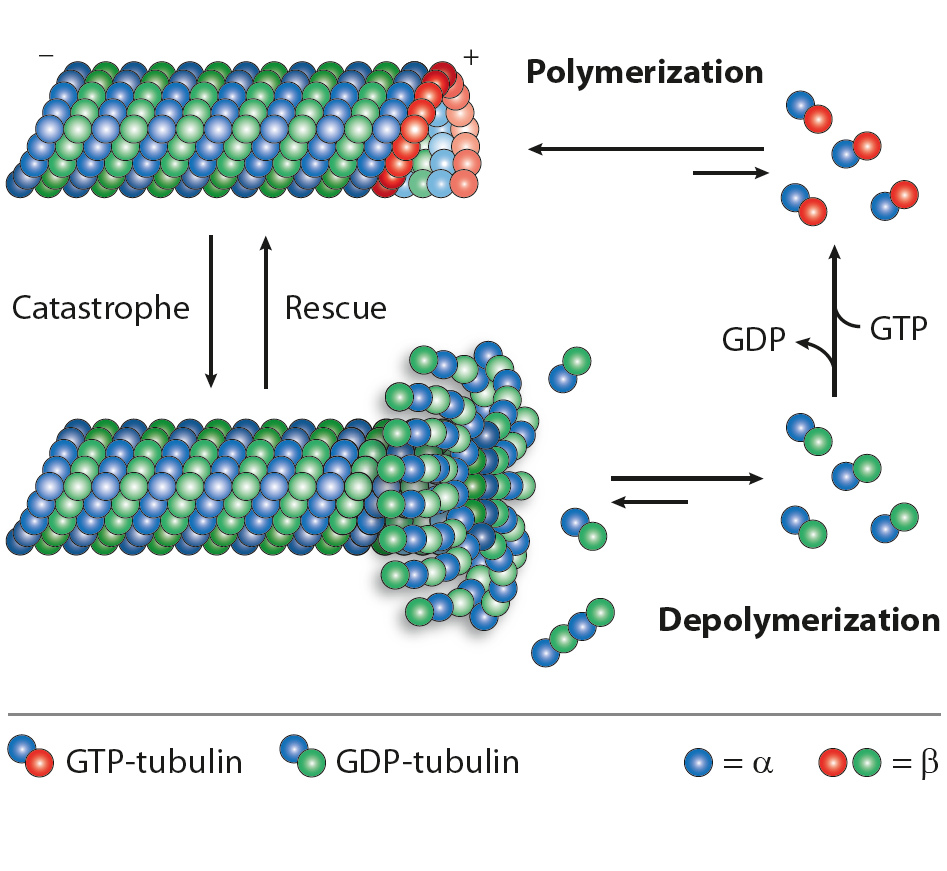
The internal organisation, shape, motility and life cycle of eukaryotic cells are all controlled by a complex network of polymeric filaments called the cytoskeleton, which includes actin filaments, intermediate filaments and microtubules. These polymers have important roles in arranging and maintaining the integrity of intracellular compartments. Microtubules are the largest cytoskeletal components involved in intracellular transport (cell signalling), cell migration/trafficking, cell division and proliferation. Microtubules control differentiative processes involving intracellular rearrangements and changes in morphology. Complex microtubule structures form the core components of centrosomes and the centrioles important for mitosis, and the core structures of cilia and flagella, which are called axonemes. Despite their functional diversity, all microtubules are assembled from heterodimers of α‑tubulin and β‑tubulin. Soluble α‑tubulin-β‑tubulin dimers polymerisation into polar microtubules in the presence of GTP. Understanding of the cell structure and function is essential for gaining a deeper knowledge of normal pathways such as morphogenesis, wound healing, neurogenesis and immune response, as well as abnormal processes such as metastasis and tumour-related angiogenesis.
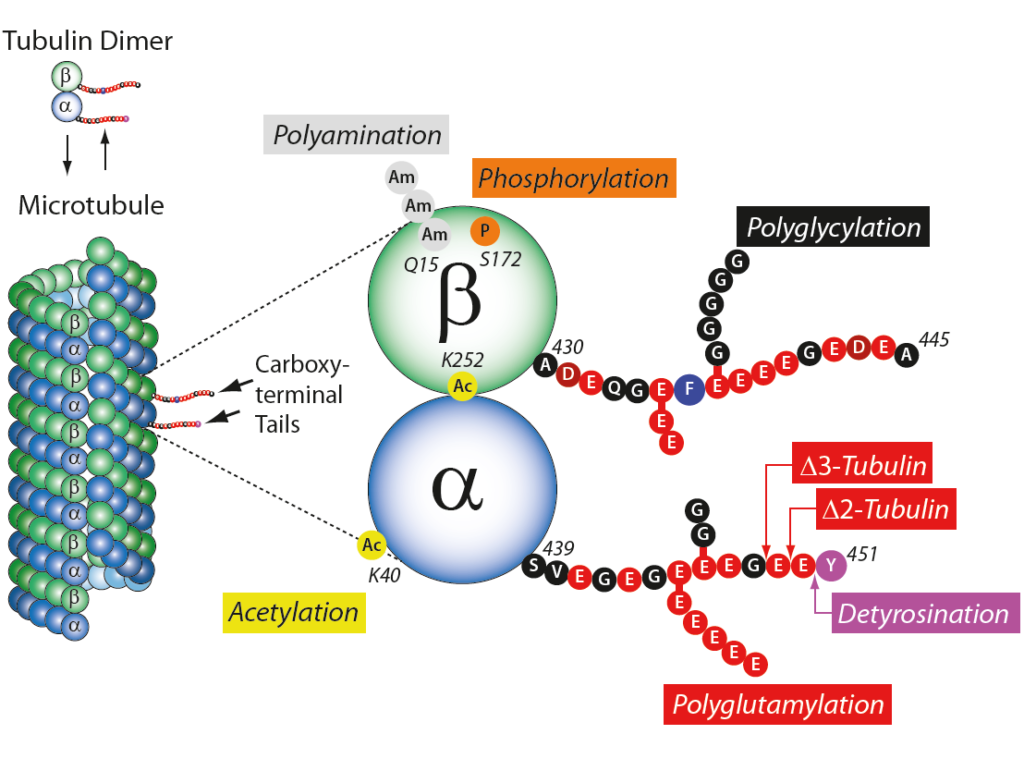
In neurons, microtubules, actin filaments and neurofilaments compose the cytoskeleton, maintaining cell polarity, architecture and morphology. Microtubules (MTs) are highly dynamic polymers formed of tubulin α and β heterodimers. Regulation of MTs polymerisation is controlled by microtubule-associated proteins, post-translational modifications of tubulin α and β, microtubules and signalling molecules. Deregulation of the neuronal cytoskeleton/MT function constitutes a key insult during the pathogenesis of nervous system diseases, leading to neurodegenerative diseases, including Amyotrophic Lateral Sclerosis, Alzheimer’s disease (AD), Hereditary Spastic Paraplegia, Parkinson’s disease (PD) and others. Posttranslational modifications (PTMs) are highly dynamic and often reversible processes where protein functional properties are altered by the addition of a chemical group or another protein to its amino acid residues. Tubulins and microtubules (MTs) are major substrates for PTMs. They include tyrosination/detyrosination, D2-tubulin formation, acetylation, phosphorylation, polyamination, ubiquitination, polyglutamylation and glycylation. PTMs are involved in fine-tuning interactions between microtubules and different MT-interacting proteins.
Literature References:
- Microtubule +TIPs at a glance: A. Akhmanova & M.O. Steinmetz; J. Cell Sci. 123, 3415 (2010)
- Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions: C. Janke & J.C. Bulinski; Nat. Rev. Mol. Cell Biol. 12, 773 (2011)
- Rab GTPases and microtubule motors: C.P. Horgan & M.W. McCaffrey; Biochem. Soc. Trans. 39, 1202 (2011)
- Rab6 is a Modulator of the Unfolded Protein Response: Implications for Alzheimer’s Disease. H.L. Elfrink, et al. ; J. Alzh. Disease 28, 1 (2011)
- The tubulin code: molecular components, readout mechanisms, and functions: C. Janke; J. Cell. Biol. 206, 461 (2014)
- Microtubules in health and degenerative disease of the nervous system: A.J. Matamoros & P.W. Baas; Brain. Res. Bull. 126, 217 (2016)
- The emerging role of the tubulin code: From the tubulin molecule to neuronal function and disease: S.Chakraborti, et al.; Cytoskeleton 73, 521 (2016)
Most Comprehensive & Unique Reagents for Microtubule Research
Validated Post-translational Modification-specific Antibodies
Tubulin-Specific Antibodies
Rab1-GTP and Rab6-GTP Specific Antibodies
Rab proteins, members of the small GTPase superfamily, are important regulators of vesicle transport via interactions with effector proteins and motor proteins. Rab1 and 6 are implicated in anterograde and retrograde trafficking in the secretory pathway. Rab1 has been shown to be involved in autophagy by helping the formation of the pre-autophagosomal isolation membrane (phagophore). Rab6 also functions as modulator of the unfolded protein response (UPR), helping the recovery from an ER stress insult. Rab6 is upregulated in Alzheimer’s disease brain.
Originally posted on adipogen.com/ptms-microtubules-neurodegeneration
Caltag Medsystems is the distributor of Adipogen products in the UK and Ireland. If you have any questions about these products, please contact us.
